In 1902, Lenard studied how the energy of the emitted photo-electrons varied with the intensity of the light. He used a carbon arc light, and could increase the intensity a thousand-fold. The ejected electrons hit another metal plate, the collector, which was connected to the cathode by a wire with a sensitive ammeter, to measure the current produced by the illumination. To measure the energy of the ejected electrons, Lenard charged the collector plate negatively, to repel the electrons coming towards it. Thus, only electrons ejected with enough kinetic energy to get up this potential hill would contribute to the current. Lenard discovered that there was a well defined minimum voltage that stopped any electrons getting through, we'll call
it Vstop. To his surprise, he found that Vstop did not depend at all on the intensity of the light! Doubling the light intensity doubled the number of electrons emitted, but did not affect the energies of the emitted electrons. The more powerful oscillating field ejected more electrons, but the maximum individual energy of the ejected electrons was the same as for the weaker field.
it Vstop. To his surprise, he found that Vstop did not depend at all on the intensity of the light! Doubling the light intensity doubled the number of electrons emitted, but did not affect the energies of the emitted electrons. The more powerful oscillating field ejected more electrons, but the maximum individual energy of the ejected electrons was the same as for the weaker field.
But Lenard did something else. With his very powerful arc lamp, there was sufficient intensity to separate out the colors and check the photoelectric effect using light of different colors. He found that the maximum energy of the ejected electrons did depend on the color --- the shorter wavelength, higher frequency light caused electrons to be ejected with more energy. This was, however, a fairly qualitative conclusion --- the energy measurements were not very reproducible, because they were extremely sensitive to the condition of the surface, in particular its state of partial oxidation. In the best vacua available at that time, significant oxidation of a fresh surface took place in tens of minutes. (The details of the surface are crucial because the fastest electrons emitted are those from right at the surface, and their binding to the solid depends strongly on the nature of the surface --- is it pure metal or a mixture of metal and oxygen atoms?)
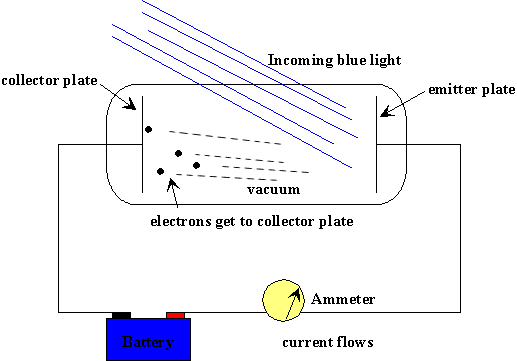
Question: In the above figure, the battery represents the potential Lenard used to charge the collector plate negatively, which would actually be a variable voltage source. Since the electrons ejected by the blue light are getting to the collector plate, evidently the potential supplied by the battery is less than Vstop for blue light. Show with an arrow on the wire the direction of the electric current in the wire.

Einstein Suggests an Explanation
In 1905 Einstein gave a very simple interpretation of Lenard's results. He just assumed that the incoming radiation should be thought of as quanta of frequency hf, with f the frequency. In photoemission, one such quantum is absorbed by one electron. If the electron is some distance into the material of the cathode, some energy will be lost as it moves towards the surface. There will always be some electrostatic cost as the electron leaves the surface, this is usually called the work function, W. The most energetic electrons emitted will be those very close to the surface, and they will leave the cathode with kinetic energy
On cranking up the negative voltage on the collector plate until the current just stops, that is, to Vstop, the highest kinetic energy electrons must have had energy eVstop on leaving the cathode. Thus,
Thus Einstein's theory makes a very definite quantitative prediction: if the frequency of the incident light is varied, and Vstop plotted as a function of frequency, the slope of the line should be h/e.
It is also clear that there is a minimum light frequency for a given metal, that for which the quantum of energy is equal to the work function. Light below that frequency , no matter how bright, will not cause photo emission.
Millikan's Attempts to Disprove Einstein's Theory
If we accept Einstein's theory, then, this is a completely different way to measure Planck's constant. The American experimental physicist Robert Millikan, who did not accept Einstein's theory, which he saw as an attack on the wave theory of light, worked for ten years, until 1916, on the photoelectric effect. He even devised techniques for scraping clean the metal surfaces inside the vacuum tube. For all his efforts he found disappointing results: he confirmed Einstein's theory, measuring Planck's constant to within 0.5% by this method. One consolation was that he did get a Nobel prize for this series of experiments.



No comments:
Post a Comment